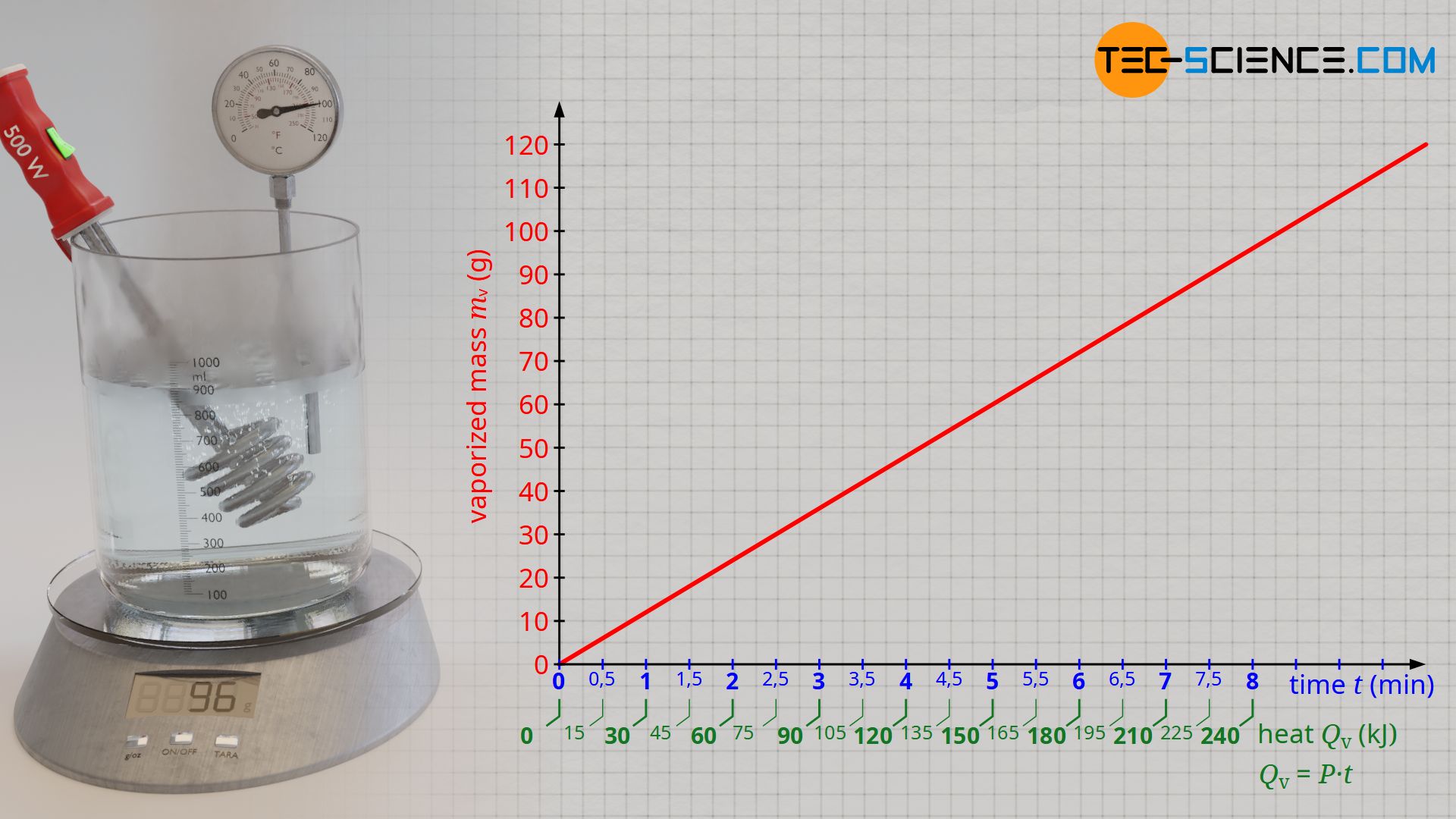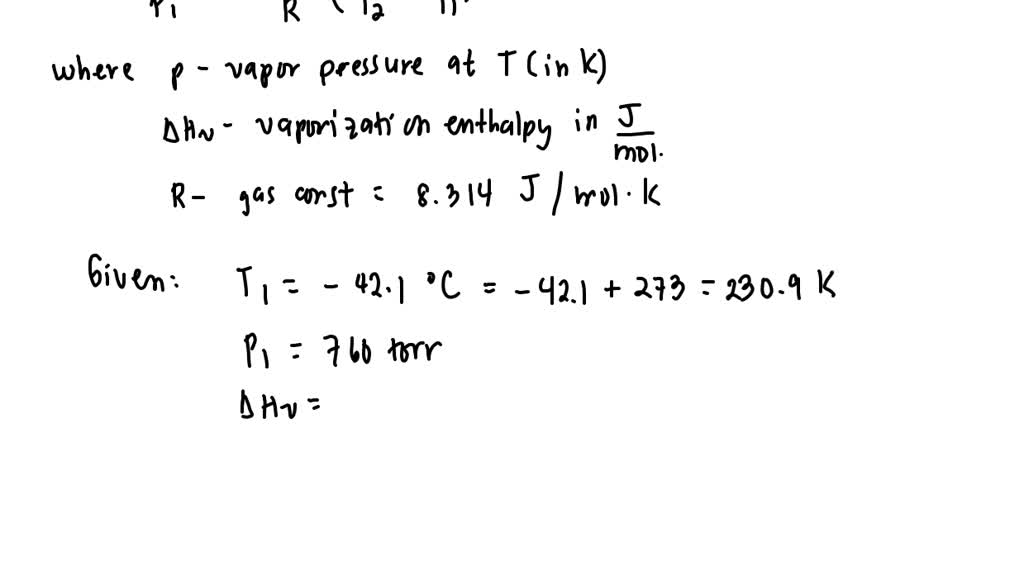
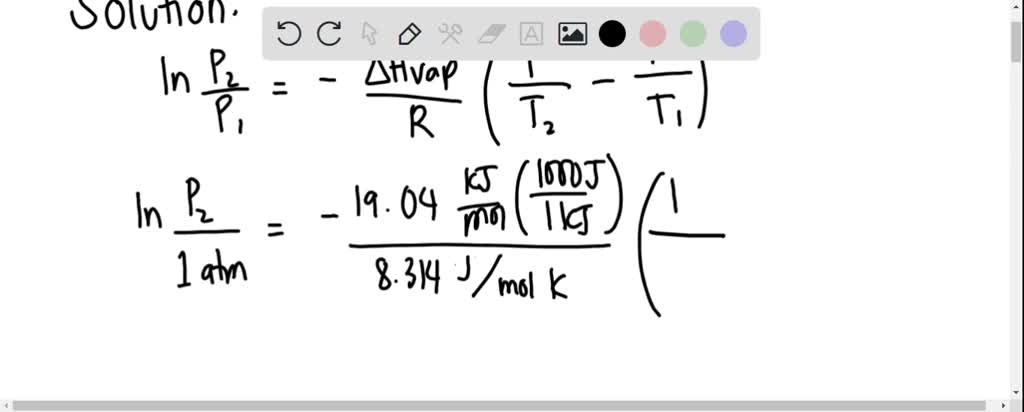
SOLVED: The enthalpy of vaporization of propane is 19.0 kJ/mol and its normal boiling point is -42.1 °C. Using the Clausius-Clapeyron equation, calculate the temperature at which propane has a vapor pressure

Energies | Free Full-Text | Heat Transfer Analysis and Operation Optimization of an Intermediate Fluid Vaporizer
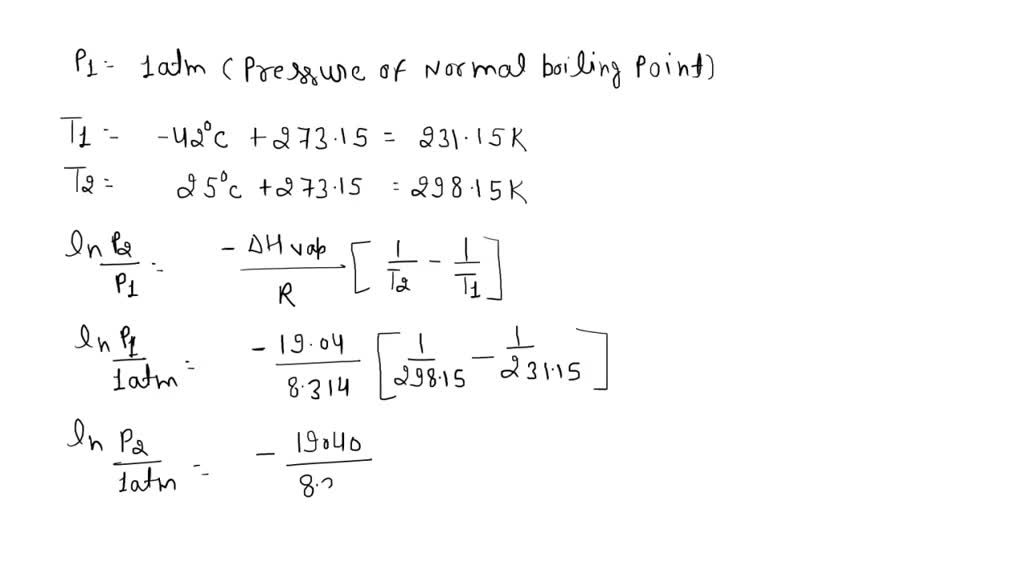
SOLVED: Propane has a normal boiling point of -42.0°C and a heat of vaporization of 19.04 kJ/mol. What is the vapor pressure, in torr, of propane at 25°C?

Table 2 from Thermodynamic Properties of Propane. II. Molar Heat Capacity at Constant Volume from (85 to 345) K with Pressures to 35 MPa | Semantic Scholar

The standard heat of combustion of propane is -2220.1 kJ/mol. The standard heat of vaporization of liquid water is 44 kJ/mol. What is the triangle H^{o} of the reaction :C_{3} H_{8} (g)+
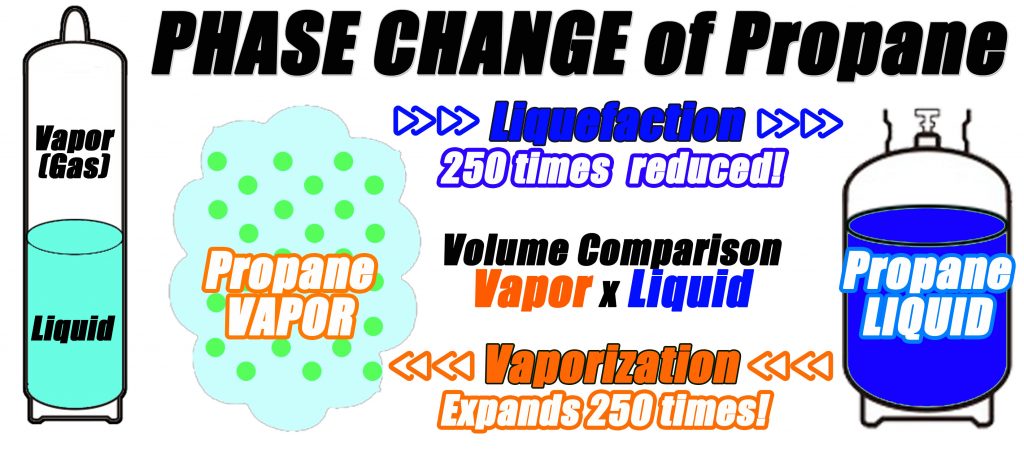
What's LPG? Why Vaporizer? – Useful Information for Users - KAGLA | Vaporizers for Propane-Butane (LPG), Ammonia and Synthetic Natural Gas

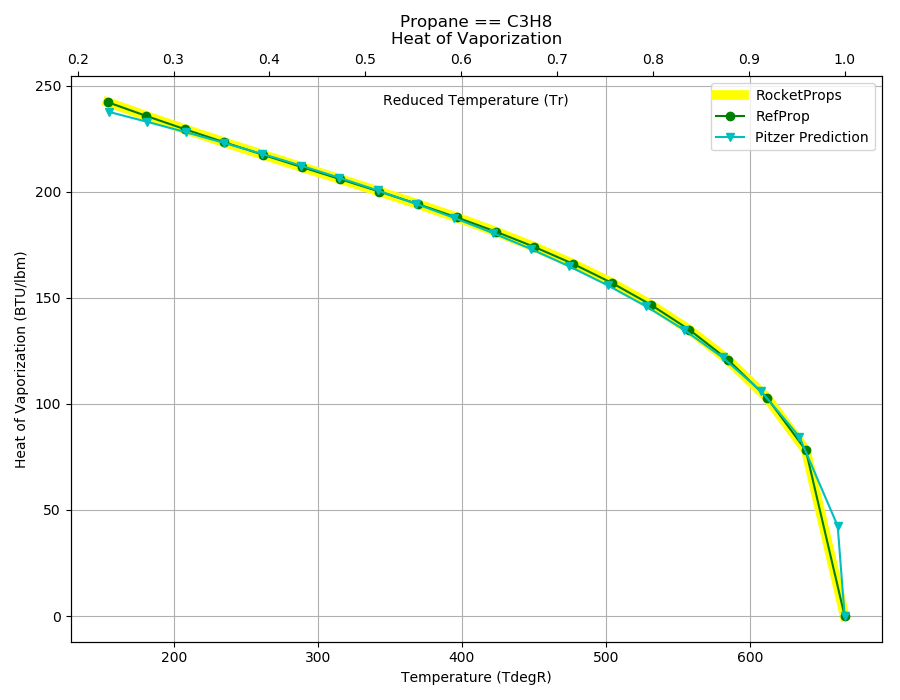
Approximated latent heat of vaporization for (a) butane; (b) isobutane;... | Download Scientific Diagram

OneClass: The heat of vaporization of propane (C3H8) is 19.4 kJ/mol. Calculate the change in entropy ...


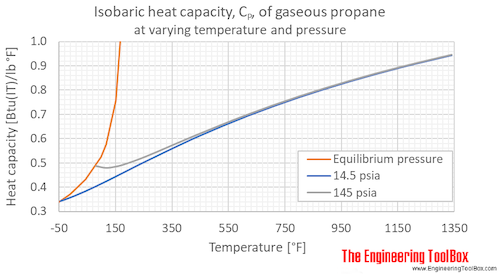
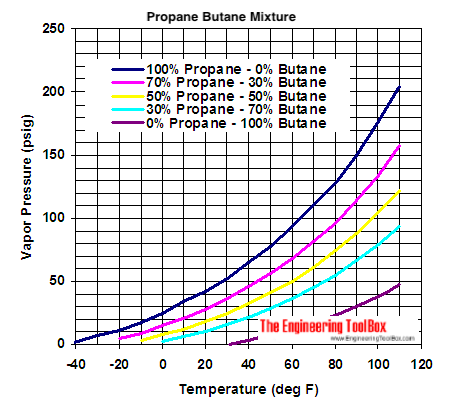
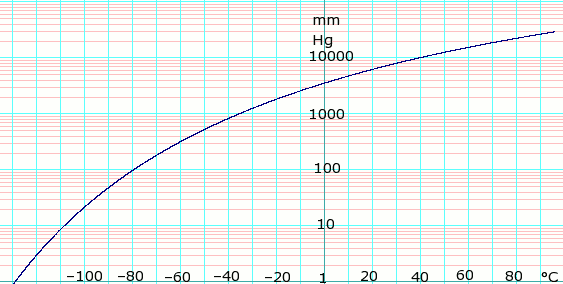
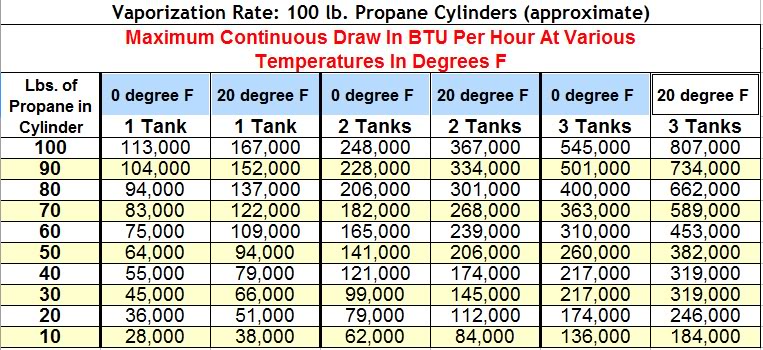

![Latent heat of vaporization for main components of LNG [10]. | Download Table Latent heat of vaporization for main components of LNG [10]. | Download Table](https://www.researchgate.net/publication/330572654/figure/tbl3/AS:718422421803010@1548296661881/Latent-heat-of-vaporization-for-main-components-of-LNG-10.png)