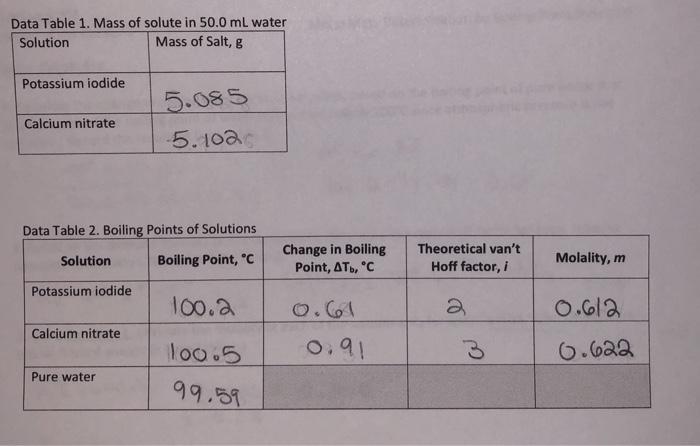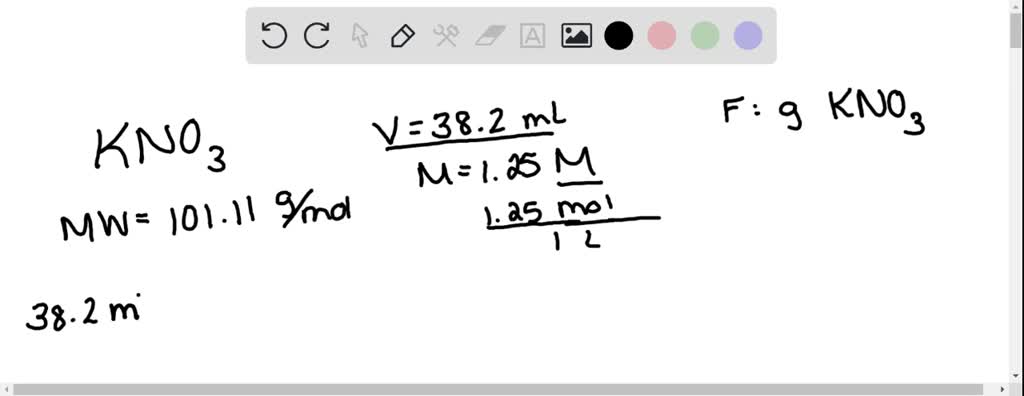
SOLVED: How many grams of potassium nitrate (molar mass = 101.11 g/mol) are in 38.2 mL of a 1.25 M potassium nitrate solution?

vuous ad Mulal Mass. elena iii) Write the chemical formulae and molecular mass of the following compounds: (a) potassium carbonate (b) calcium chloride c) Sodium nitrate d) Acetic acid i) An element

MOLE (mol) Mass (g) Particles (atoms,m'c or f.u.) DO NOW: Copy the conversion factors that allow you to convert from mass to moles and from particles. - ppt download

SOLVED: How many grams of potassium nitrate are required to prepare 0.250 L of a 0.700 M solution? 2.8 g 101.0 g 0.175 g 17.7 g

SOLVED: Question 11 What is the molar mass of potassium nitrate (KNO 3)2 Oa 178.05 g mol 6.122.0 g mol 101.1 g mol d. 84.99 g mol 91.19 g mol

MOLE CONVERSIONS Which conversion factors allow you to convert from mass to moles and from particles to moles. molar mass (g) . 1 mol x 1023 particles. - ppt download

What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water st 313 K?
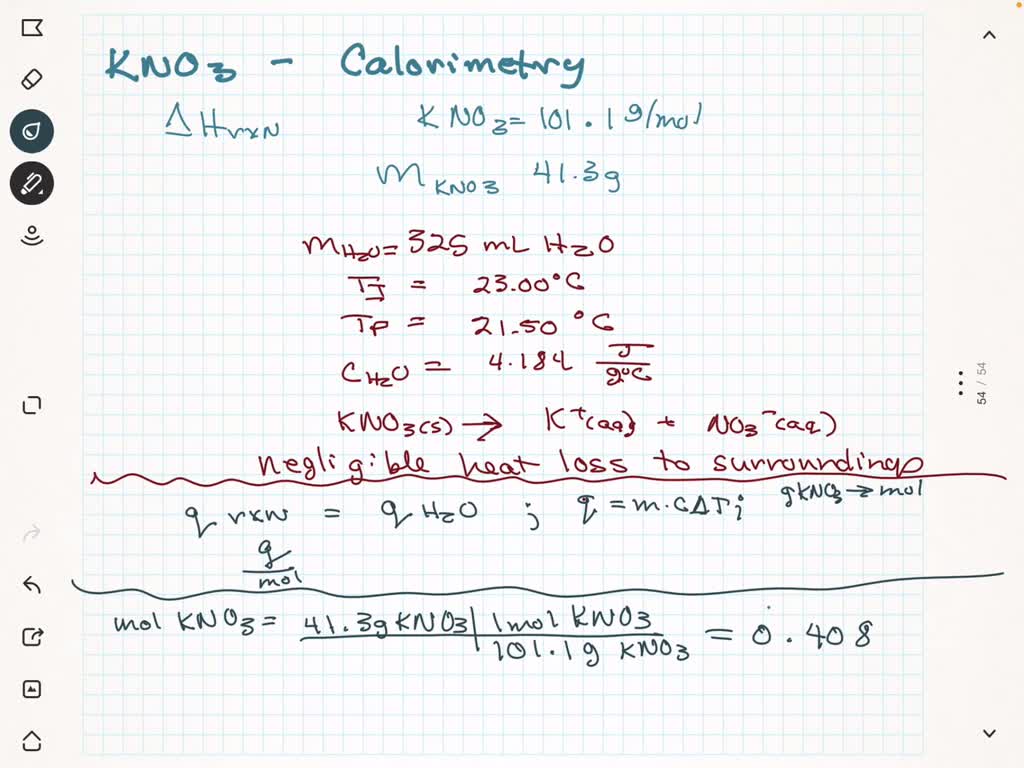
SOLVED: Potassium nitrate, KNO3, has a molar mass of 101.1 g/mol. In a constant-pressure calorimeter, 41.3 g of KNO3 is dissolved in 325 g of water at 23.00 °C. KNO3(s) â†' K+(aq) +
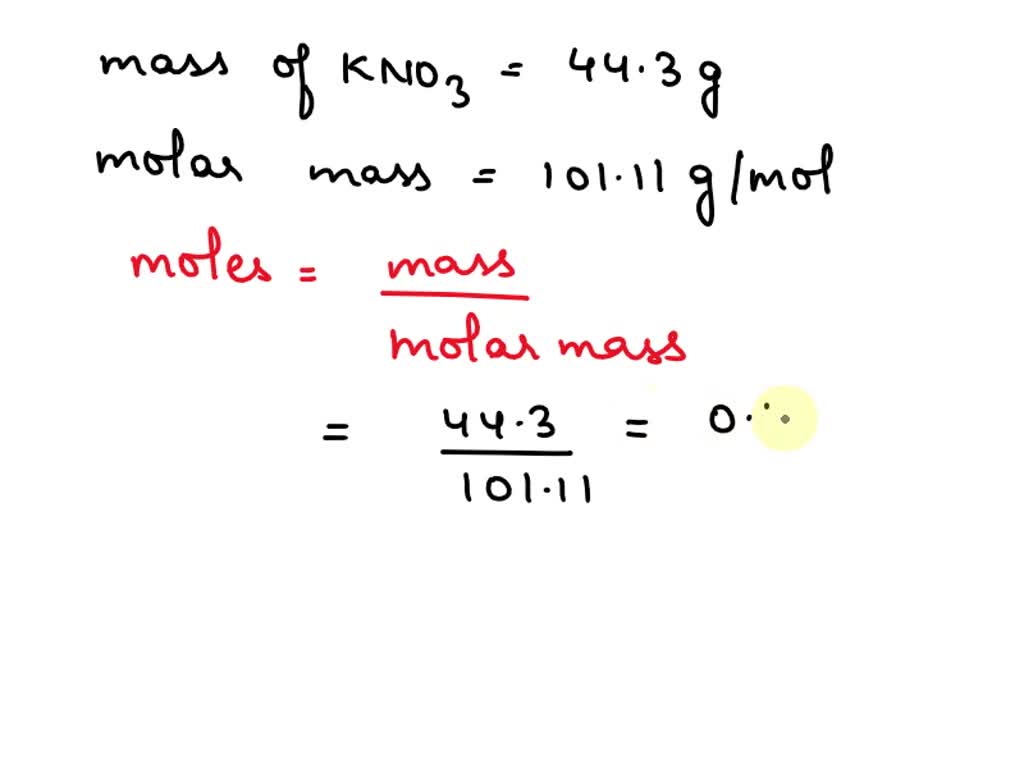




![Potassium nitrate [KNO3] Molecular Weight Calculation - Laboratory Notes Potassium nitrate [KNO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/potassium-nitrate-molecular-weight-calculation.jpg)