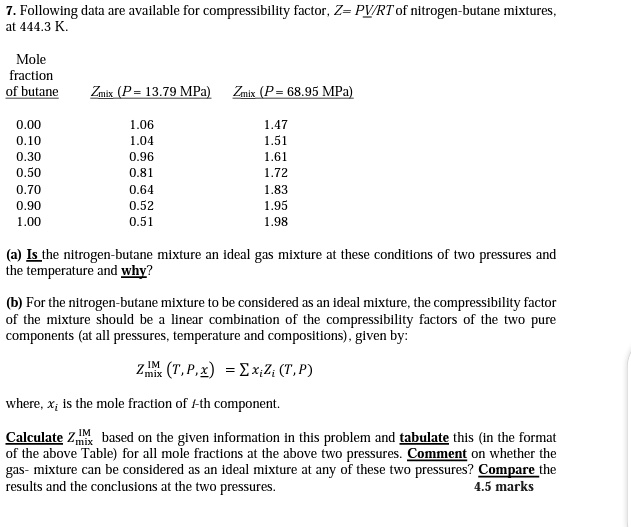
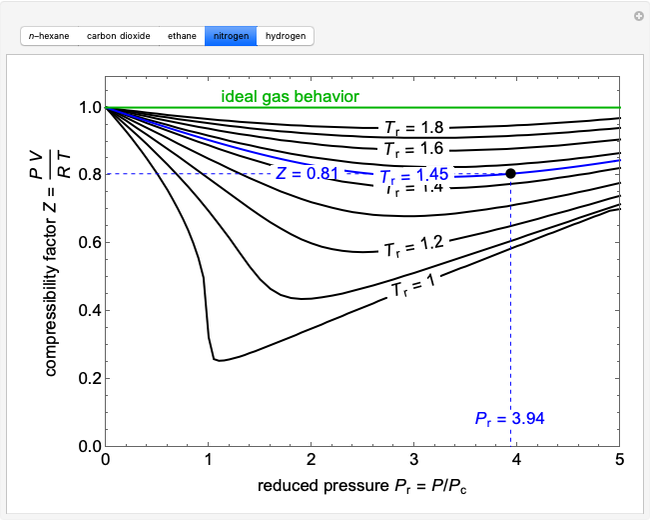
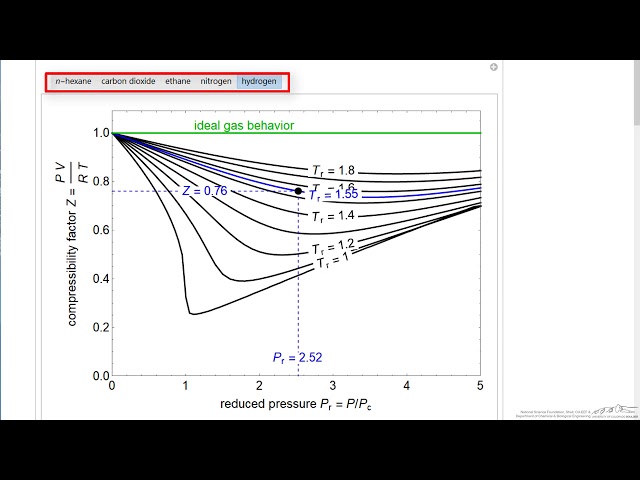
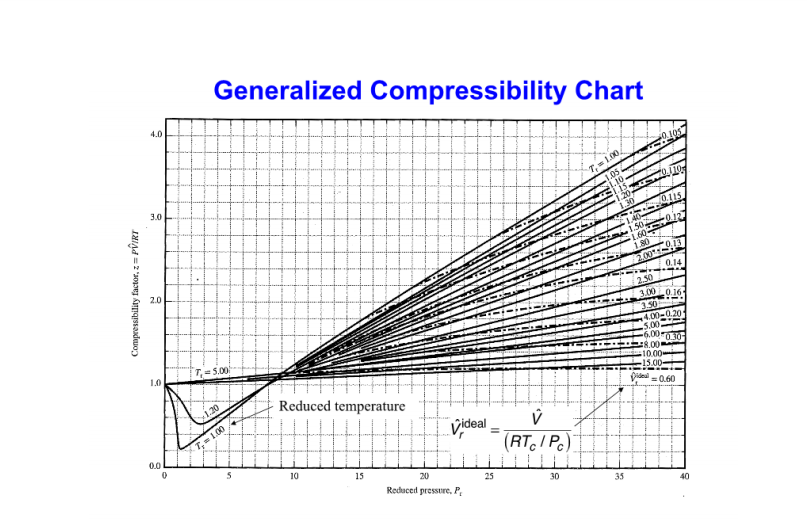
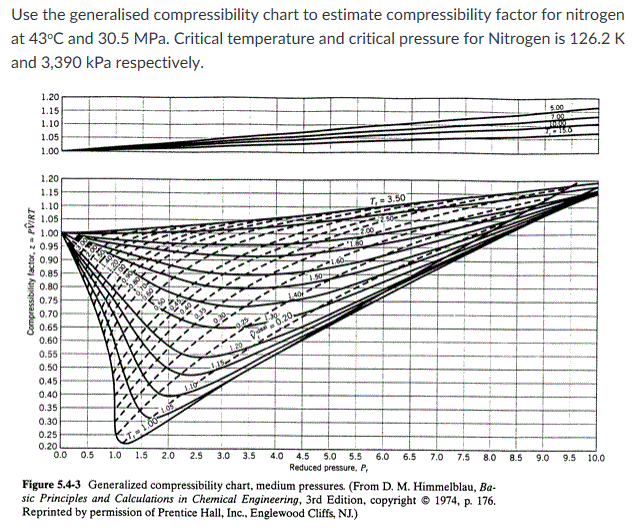
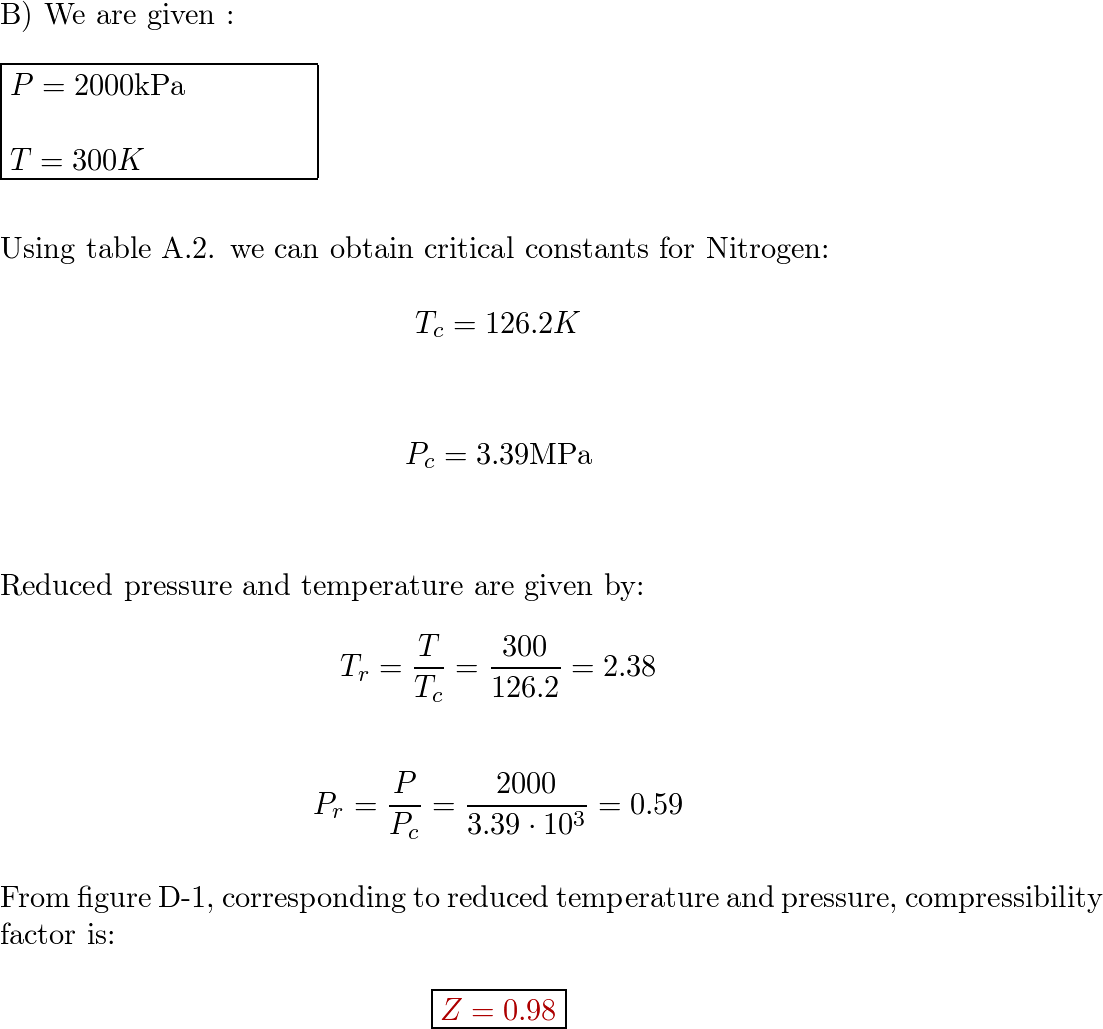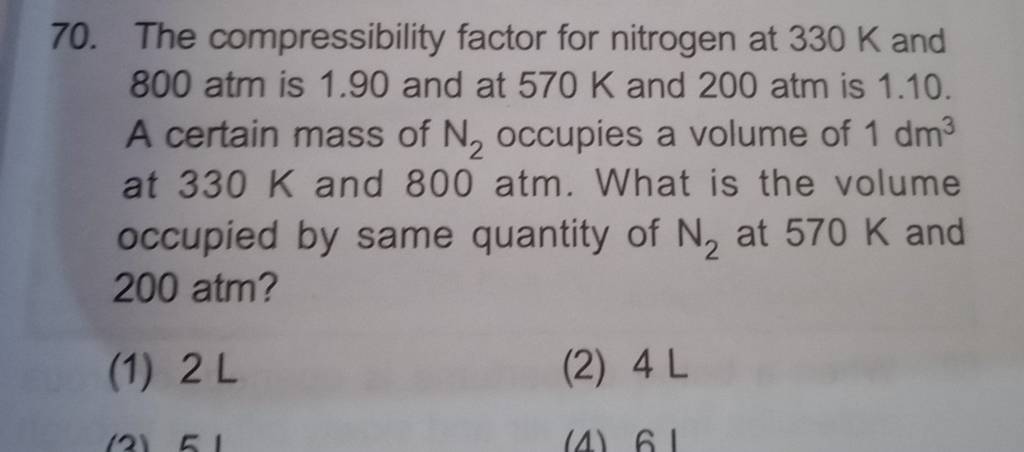physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

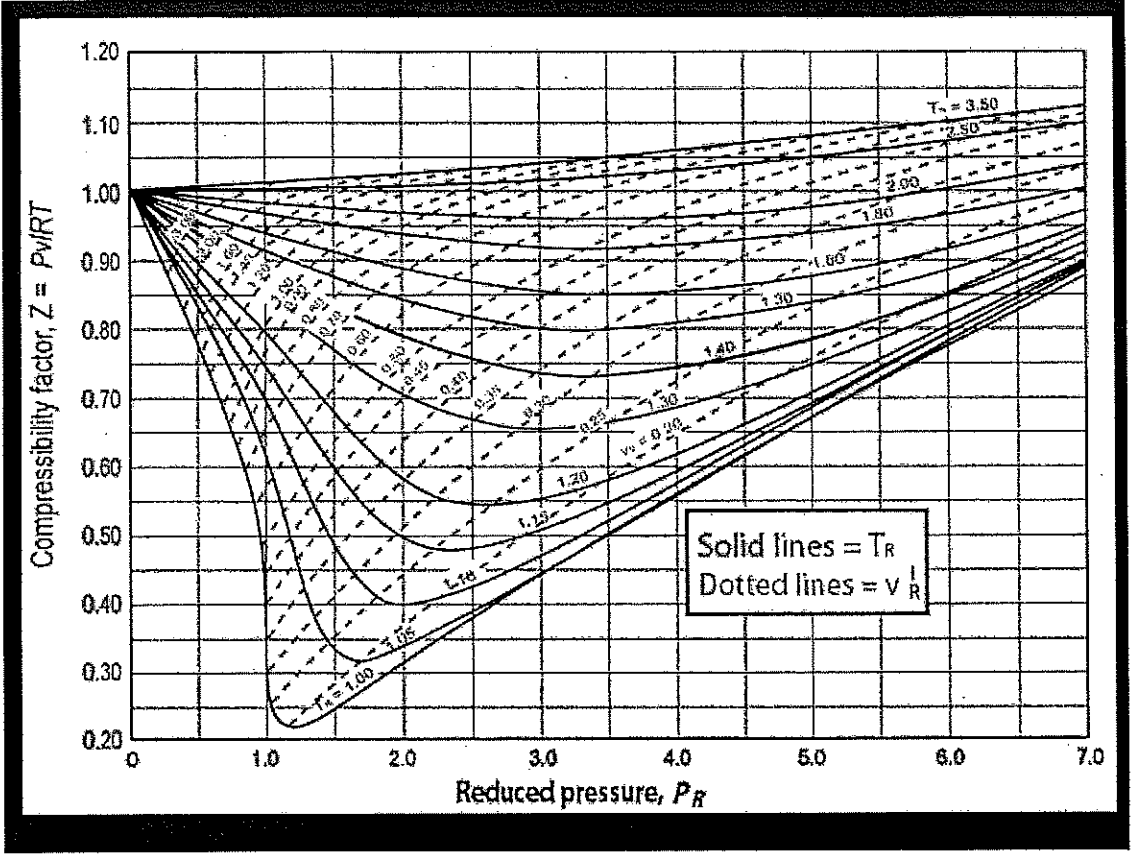
Figure 7 from A Simple Equation Of State For Calculating The Compressibility Factor Of Pure Fluids Based On The Virial EOS | Semantic Scholar

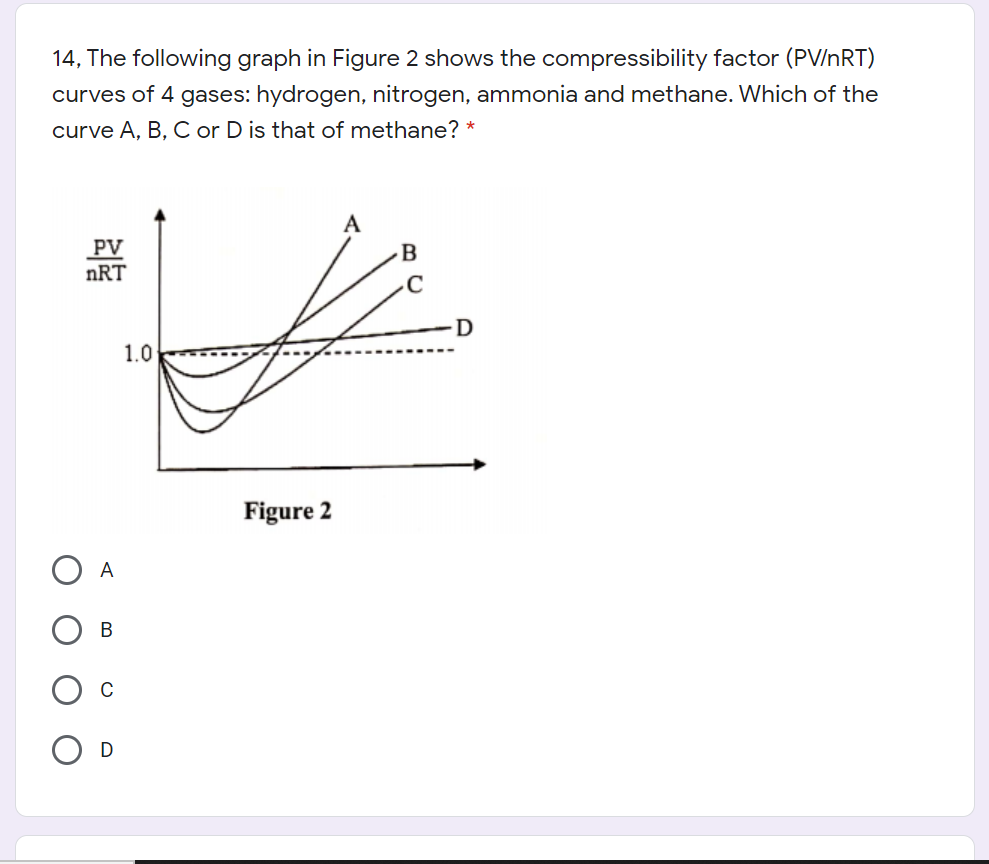
physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange

Sensitivity and compressibility of nitrogen at 4.0 MPa. The absolute... | Download Scientific Diagram
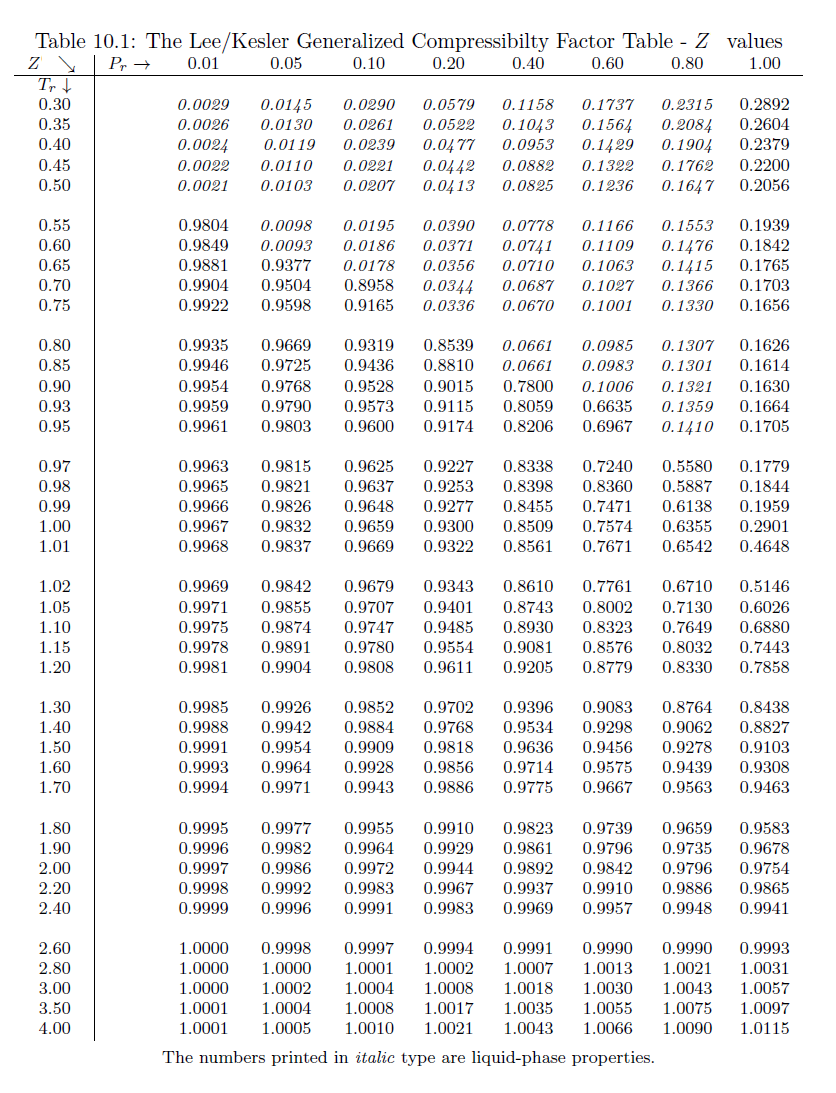
![PDF] Charts of Compressibility Factors and Charts Showing Quantities Delivered by Commercial Cylinders for Hydrogen, Nitrogen, and Oxygen | Semantic Scholar PDF] Charts of Compressibility Factors and Charts Showing Quantities Delivered by Commercial Cylinders for Hydrogen, Nitrogen, and Oxygen | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0c6768d49e1dc41774af30c3afeb368ba6bda3bf/5-Table1-1.png)
PDF] Charts of Compressibility Factors and Charts Showing Quantities Delivered by Commercial Cylinders for Hydrogen, Nitrogen, and Oxygen | Semantic Scholar