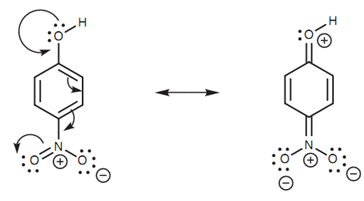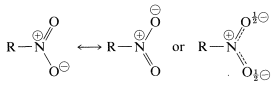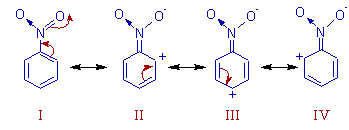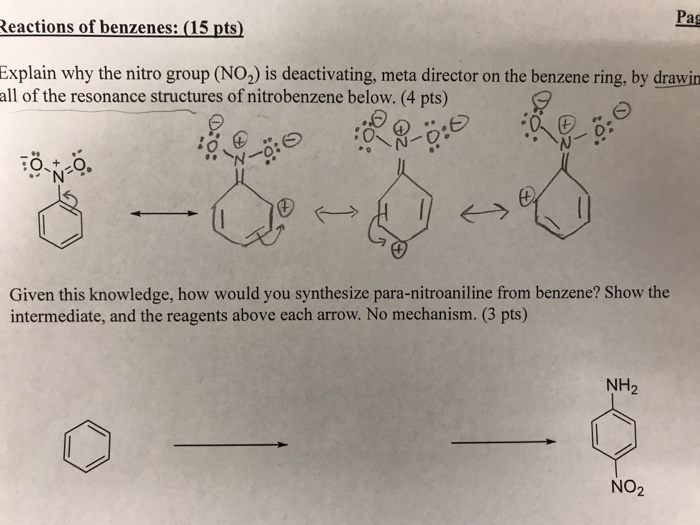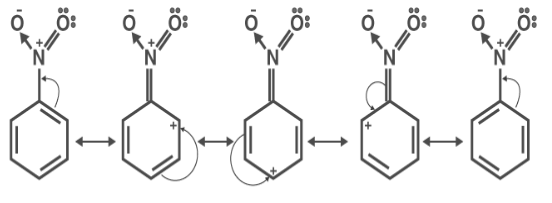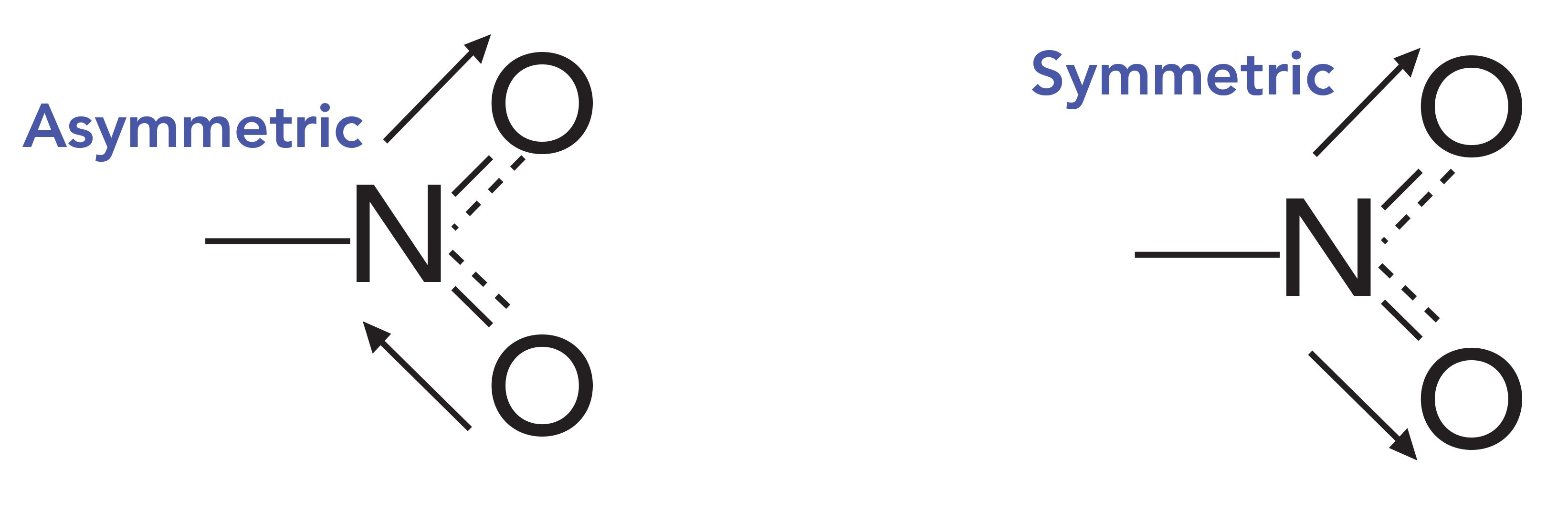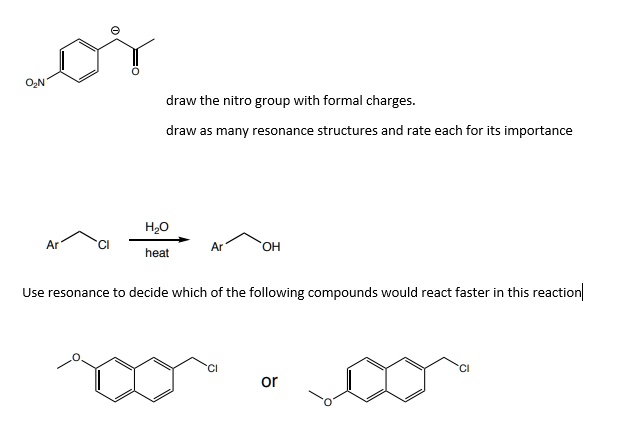
SOLVED: draw the nitro group with formal charges draw as many resonance structures and rate each for its importance HzO OH heat Use resonance to decide which of the following compounds would
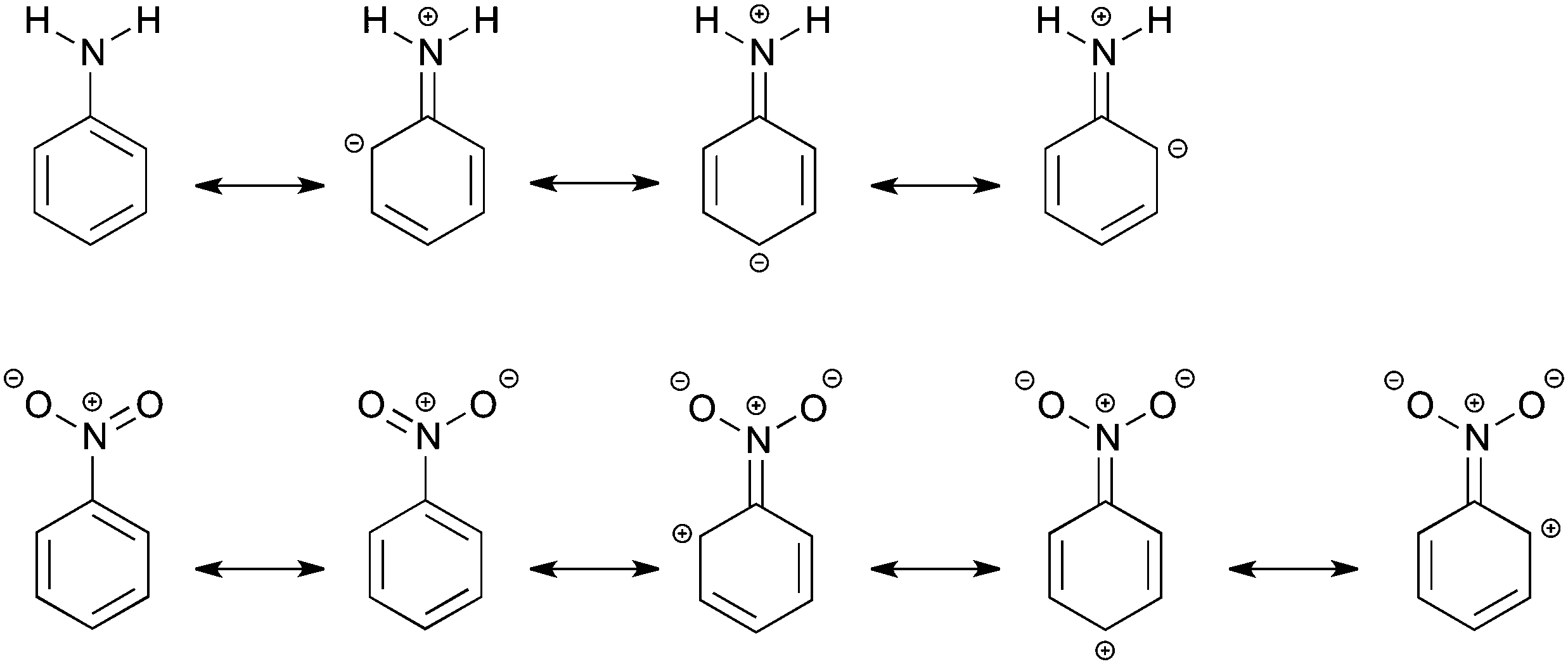
How amino and nitro substituents direct electrophilic aromatic substitution in benzene: an explanation with Kohn–Sham molecular orbital theory and Vor ... - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C5CP07483E

Pharmaceuticals | Free Full-Text | The Diverse Biological Activity of Recently Synthesized Nitro Compounds
Title: Draw the Resonance Structure of Nitro Benzene- (For CBSE, ICSE, IAS, NET, NRA 2024) FlexiPrep

Show resonance of the 2-nitrophenoxide ion which explains why 2-nitrophenol is stronger acid than phenol. | Homework.Study.com
Explain the directive influence of nitro group in nitrobenzene. - Sarthaks eConnect | Largest Online Education Community