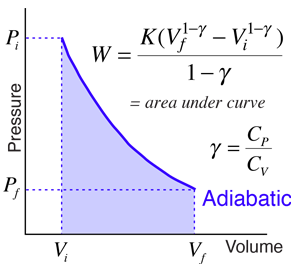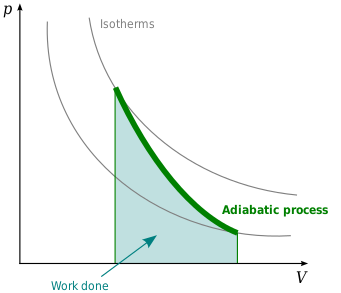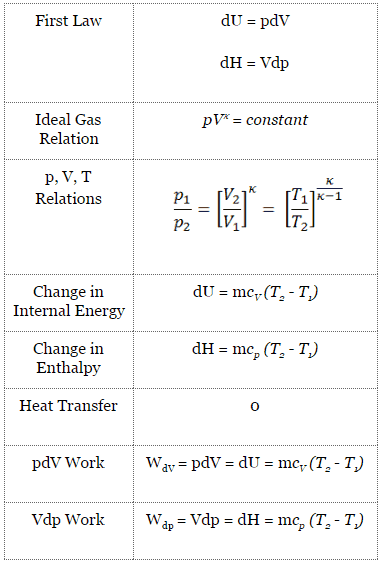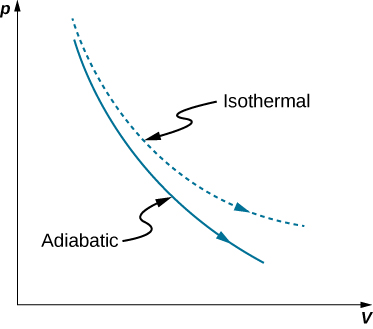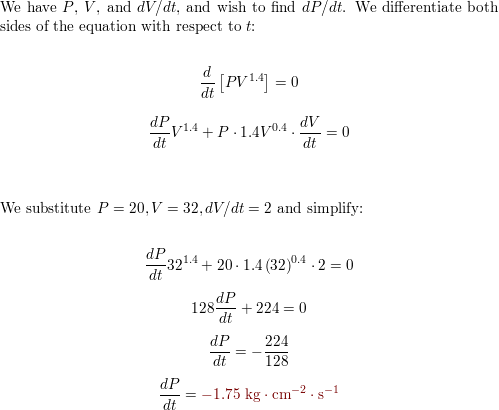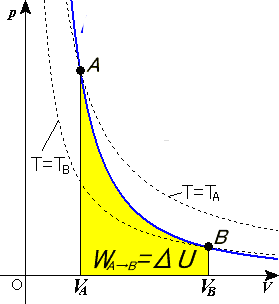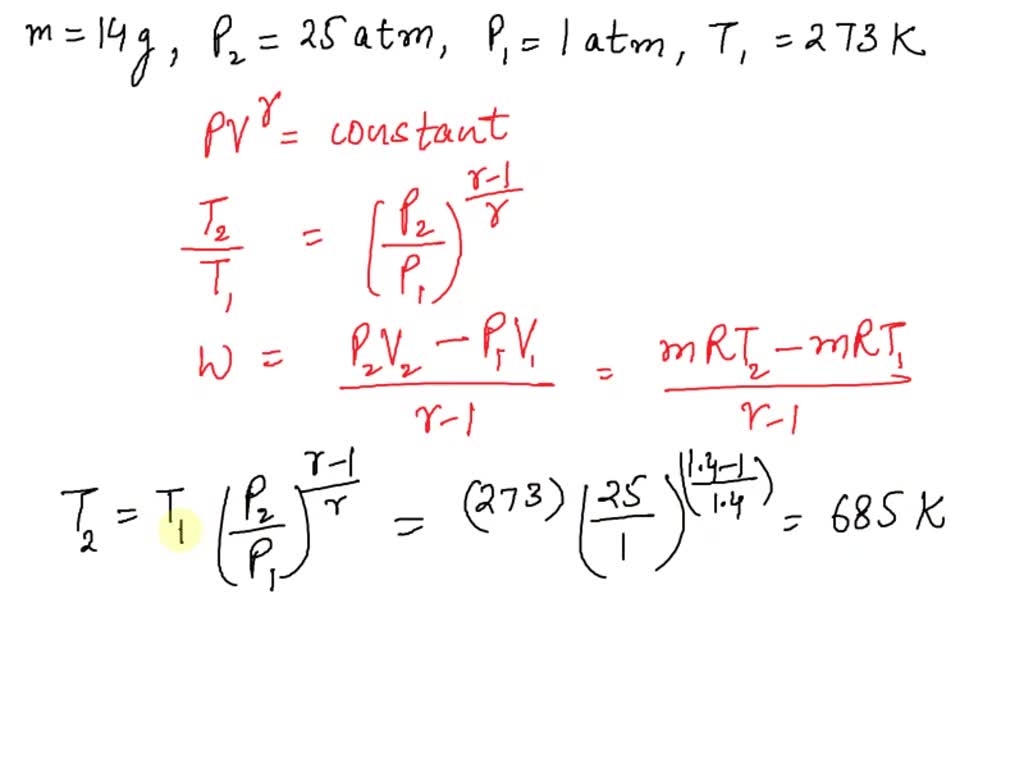
SOLVED: 14 g of nitrogen gas at STP are adiabatically compressed to a pressure of 25 atm . What is the final temperature? What is the work done on the gas? What
When an ideal gas is compressed adiabatically and reversibly,the final temperature is 1 higher than the initial temperature 2 lower than the initial temperature 3 same as the initial temperature 4 dependent

Grade 11 || 1st Law of Thermodynamics || 2071 'C' || Explanation | Grade 11 || 1st Law of Thermodynamics || 2071 'C' || Explanation Qus: Air is compressed adiabatically to half its volume. Calculate the change in its... | By Satsom | Facebook

Air is first compressed adiabatically from 100 kPa and 300 K to 1500 kPa and a specific volume of 0.1 m3/kg. The air is then cooled at constant volume back to 300
A gas (γ = 1.5) is compressed in an adiabatic process, then its volume changes from 1600 cm^3 to 400 cm^3. - Sarthaks eConnect | Largest Online Education Community

Argon gas is adiabatically compressed to half its volume. If P, V and T represent the pressure, ... - YouTube
An ideal gas is adiabatically and irreversibly compressed from 3 bar and 300 K to 6 bar in a closed system. The work required for the irreversible compression is 1.5 times the

When a certain mass of an ideal gas is adiabatically compressed 1 Ideal gas is adiabatically compressed so that its volume is reduced to 1/32 atoms in a molecule (atomicity) times, its

physical chemistry - Isothermal vs. adiabatic compression of gas in terms of required energy - Chemistry Stack Exchange

Applied thermodynamics for engineers. ig. to. Arts. 205, 206. —Two-stage Com-pressor Indicator Diagram. a second cylinder, is adiabatically compressed along EF^ ejected andcooled along FCr^ and finally compressed in still another

A liquid is adiabatically compressed from state I to state II suddenly by a single step, as shown in the figure then

T-S diagram of high temperature adiabatic compressed air energy storage... | Download Scientific Diagram
![PDF] Adiabatically compressed wave dark matter halo and intermediate-mass-ratio inspirals | Semantic Scholar PDF] Adiabatically compressed wave dark matter halo and intermediate-mass-ratio inspirals | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d1367a951cd0b35d0b8186467936e41f21fa24f3/5-Figure1-1.png)
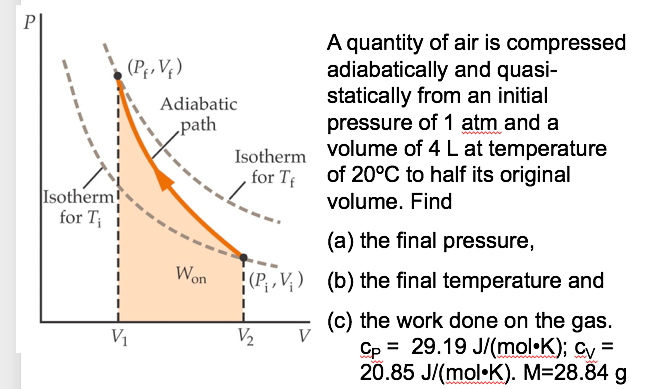

![Bengali] Some amount of air, initially at STP, is adiabatically compr Bengali] Some amount of air, initially at STP, is adiabatically compr](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/3845343.webp)